From supply chain issues causing mask shortage to counterfeit production— where did it all go wrong?
Since the start of the global pandemic, the demand for personal protective equipment or PPE has skyrocketed. With only PPE in between a person and the infection, this has been an inevitable consequence of the pandemic. The increased demand opened doors for supply chain issues in all countries. Having COVID-19 hit China earlier in the year combined with having majority of PPE manufacturers centralized in China, majority of the PPE back up supply was greatly reduced without increasing future production.
Back in February, United States’ mask production would make 200,000 a day while China was operating at a 76% capacity with 15 million pieces a day. This falls short to the demand of 50 million masks a day in China alone. Different countries have also restricted exports to protect their domestic supply. Shipments have also been limited due to decreased air travel and slow clearing in customs. All these factors have caused price gauging on all selling platforms with masks going up to more than a hundred dollars a pack.
To combat this dilemma, hundreds of new companies were established in China to respond to the rising demands. In weeks’ time, companies that do not normally produce masks has opened their factories to aid the global mask shortage. Regulation of these companies have been questioned yet many out there are still operating without actual proof of legitimacy.
Normally, PPE and respirators used in the US are approved by the National Institute for Occupational Safety and Health (NIOSH) and the FDA. Not all masks and respirators need to be cleared by the FDA. Masks that do not prevent infection or have antiviral/microbial properties do not need clearance. Due to the mask shortage, FDA released a policy stating manufacturers can request for an Emergency Use Authorization (EUA.) This EUA would allow importing of non-NIOSH approved N95 respirators that had undergone evaluation and certification like NIOSH standards. Countries included were Australia, Europe and Japan to name a few. China was recently added to the list making KN95 respirators eligible for authorization although it is frequently being updated due to numerous fraudulent claims of certification. FDA released a list of authorized non-NIOSH approved respirators made in these countries along with its manufacturers to help guide consumers. But despite all these measures, counterfeit n95 respirators and other PPE are continuously being sold and used even in healthcare settings.
According to the CDC, the three most common scenarios that pave way to procurement of counterfeit products are the following:
- Certification marks are counterfeit— Review of companies based in China had revealed significant number of falsified certifications claiming that they are FDA certified. Due to this, companies must not rely solely on this as basis for the credibility of the product.
- Documents are altered to conform with the standards of approval- Respirators from authorized manufacturers always undergo testing and quality control. Performance characteristics such as filter efficiency and other physical components are recorded to determine if the respirators are of a certain standard. Without this, counterfeit masks do more harm than it does any good by giving the consumers a false sense of security.
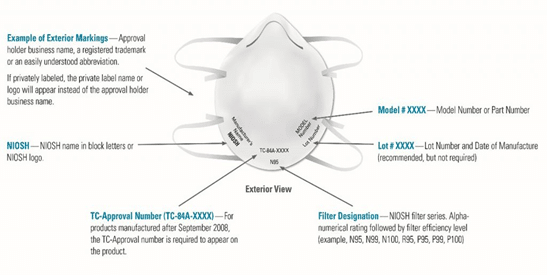
- Company names, logos and model numbers are counterfeit– NIOSH has released an image to guide buyers to determine if their mask is counterfeit or not. To verify NIOSH approval, the most important thing to note is the “TC Number.” Each TC number can be verified on the NIOSH certified equipment list. NIOSH also offers filtration efficiency testing in respirators purchased outside the US. An updated guide of NIOSH approved respirators can be found here.
Moreover, logistics has also played a valuable role in the flooding of counterfeit products. Orders are done in bulk—in thousands to millions where no samples are shipped. Pre-payment is usually required before an order is placed. And since China has closed its doors for travel, buyers from other countries cannot inspect the quality of the masks firsthand. With quality control greatly compromised, it has led to the fear of procuring China made masks due to liability issues.
All these factors have greatly impacted the global PPE shortage and a direct cause of increase in COVID19 cases worldwide. What is the light at the end of tunnel? We hope as months pass by, countries and companies have adapted to the situation and are able to provide more efficient solutions and improved regulations. For now, consumers must do their part, be extra vigilant and do its due diligence before purchasing.

